Articles and Blogs
Share:
Maintaining controlled environments is at the heart of pharmaceutical manufacturing, biotechnology processes, and biosafety research. Among the various cleanroom parameters, room pressure and differential pressure monitoring play a decisive role in ensuring product integrity, contamination control, and compliance with regulatory guidelines.
Importance of Room Pressure Control in Cleanrooms
In cleanrooms, air must always move in a controlled direction. For pharmaceutical cleanrooms, this usually means maintaining positive pressure so that clean air pushes into less clean adjoining spaces, preventing external contaminants from entering. On the other hand, in biosafety labs handling hazardous materials, negative pressure ensures contaminants stay contained.
Without reliable room pressure monitoring systems, cleanrooms cannot consistently meet pharma cleanroom requirements, and even small fluctuations can compromise product quality and safety.
Differential Pressure Monitoring Between Classified Areas
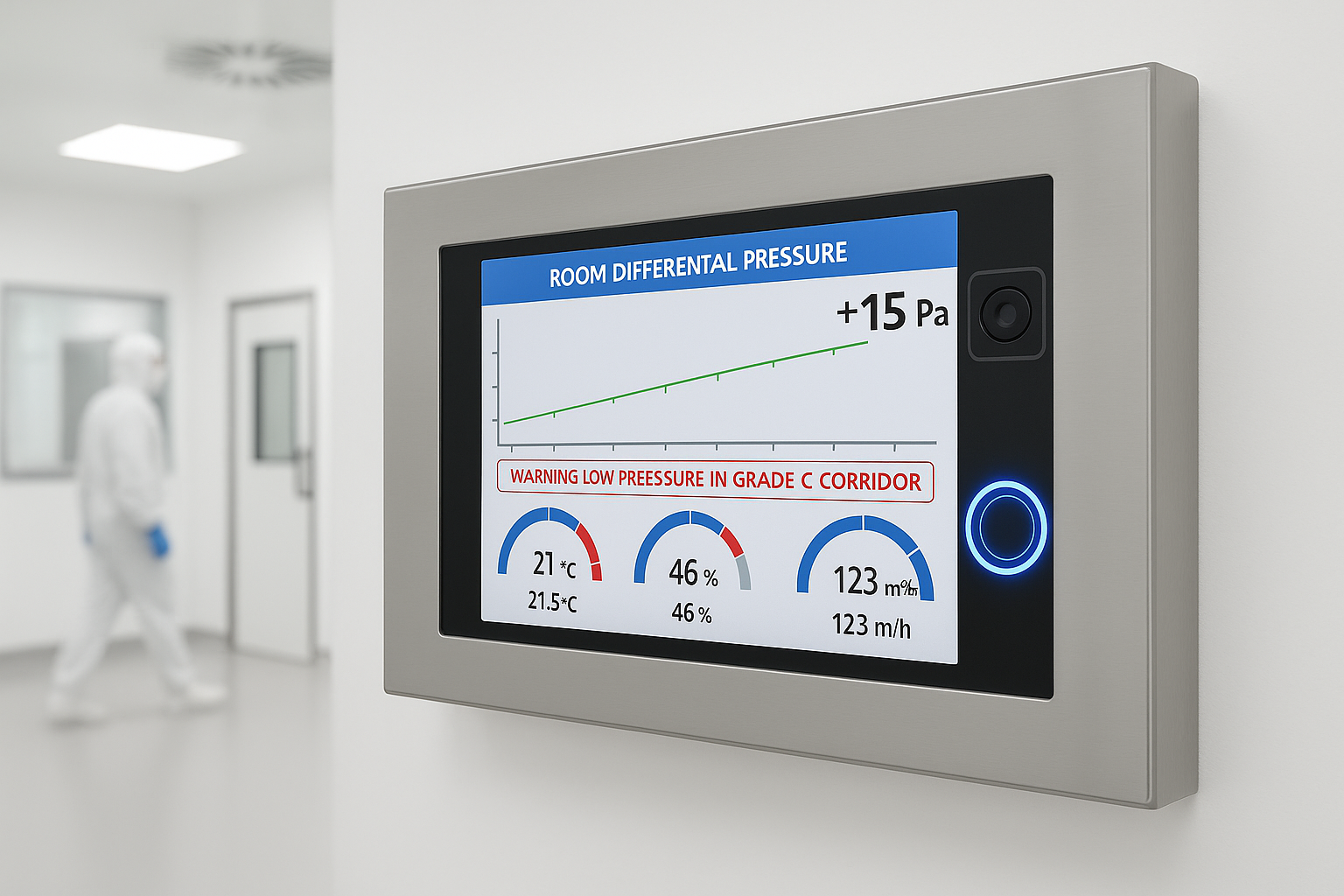
Every cleanroom facility is designed with graded zones—such as Grade A, B, C, or D in pharmaceuticals—each with its own cleanliness level. A room differential pressure monitor helps track the pressure difference between these zones to maintain the correct airflow direction.
For example, between a Grade B cleanroom and a Grade C corridor, a minimum differential pressure must be maintained to prevent the inflow of particles. Monitoring ensures these cascades remain intact and alarms operators instantly if a deviation occurs.
Risks of Improper Pressure Maintenance
Not controlling room pressure effectively can lead to:
- Cross-contamination – allowing particles, microbes, or aerosols to travel into cleaner zones.
- Batch failures and product loss – leading to costly rejections or recalls.
- Regulatory non-compliance – with agencies like the FDA, WHO, or EU GMP issuing warnings or halts in production.
Such risks highlight why a robust clean room monitoring system is an investment in both compliance and quality assurance.
Key Features of an Effective Monitoring System
Modern cleanroom monitoring equipment goes beyond simple gauges. A good system should provide:
- Continuous real-time pressure display for operators.
- Visual and audible alerts for immediate response to deviations.
- Data logging and reporting to simplify audits and documentation.
- Integration with BMS or EMS for centralized facility control.
- User-friendly dashboards to make monitoring intuitive for staff.
These features not only ensure adherence to regulatory standards but also make daily operations smoother and safer.
Applications Beyond Pharma
While pharmaceuticals are the most stringent users, differential pressure monitoring is equally vital in:
- Biotech manufacturing – ensuring aseptic processing.
- Biosafety laboratories (BSL-3/BSL-4) – where negative pressure safeguards researchers.
- Hospitals and healthcare facilities – particularly isolation rooms and surgical theaters.
Enviro Technologies: Trusted Cleanroom Monitoring Partner
At Enviro Technologies, we specialize in delivering reliable room pressure monitoring systems and differential pressure monitors tailored to pharma and biotech cleanrooms. Our solutions are designed for accuracy, compliance, and ease of integration with existing systems. With years of expertise in cleanroom monitoring equipment, we help facilities maintain regulatory standards while ensuring patient and product safety.
Whether it’s a pharmaceutical plant, biosafety lab, or biotech facility, Enviro Technologies provides the tools and support you need to keep your cleanrooms compliant, efficient, and secure.

Head-BFSI